Article from European Magazine: Galvanotechnik 3/2013
Diamondyze: A Breakthrough in Aluminium Anodising?
Dr. A. T. Kuhn
Is it really possible that after almost a century, we could be seeing a significant advance in aluminium anodising ? An American, Leonard Warren [2], of Tech-Line Coatings, with several decades experience in the coatings industry and a Canadian Metal Finisher, Dale Hupe [3] (Hupe Manufacturing), have developed the “DiamondyzeTM Process” , also described as “Ceramic Anodising” and believe they have done just that.
The first stage of their new anodising process is more or less conventional, using an aqueous acid electrolyte. Their claimed breakthrough comes at the following stage, where the freshly anodised aluminium is immersed in a solution containing an undisclosed additive. This step is very much the same as the classical dye coloring process for anodised aluminium, and indeed a colouring dyestuff can be included, although it is not necessary, in which case the anodised aluminium remains the same colour as it was immediately after anodising.
Finally, the surface can be sealed using conventional nickel acetate, hot water, steam or other means, though as we see below, this may not be necessary. The result is an extremely hard and corrosion and wear-resistant anodic oxide coating, as detailed below.
Is this new process patented? Perhaps surprisingly, at least at first sight, the answer is no. But as co-inventor Leonard Warren explained, this is by deliberate choice. As he rightly notes, in an industry dominated by giant aerospace and marine corporations, a small company can all too easily be battered into submission by sheer weight of legal aggression. The inventors have a further valid reason for not going down the patent route, as we shall see.
So what lies at the heart of the new process? The answer lies in the complex, and not yet fully understood chemistry taking place in Stage 2. In a short online video [1] it is noted that ceramic particles in the additive are impregnated into the anodic oxide alumina pores. The additive acts to constrict the aluminium oxide pores, to oxidise any metallic aluminium not previously oxidised in the electrochemical process, and also to largely fill the porous structure.
This is one reason why final sealing becomes less important than in conventional anodising. Without revealing the sophisticated chemistry involved in this, the inventors are confident that it is so complex that there is no threat to their invention from “reverse engineering”, should a sample of the new coating fall into the wrong hands. While they understandably do not reveal the chemical nature of the additive, they note that it is added to the immersion solution in small amounts, of order 2.5 ml additive per litre water. The immersion solution adopts the pH of the dyestuff used, in absence of dyestuff, pH is approx 6.
The developers are also at pains to emphasise the absence of any toxic or environmentally undesirable chemicals in their process. Figure 1 shows a metallographic cross-section of a Diamondyze coating over an aluminium piston.

So what can be done with the new process? Firstly, it appears to operate with a very wide range of aluminium alloys, including cast aluminium and the commercially important types such as 2024 and 6061. On these and others, Type II and Type III anodising can be carried out. Next, it is reported that anodised surfaces from the new process have far lower roughness than conventional anodising, indeed surface roughness is usually less than that of the original unanodised
aluminium substrate. Figure 2 shows surface roughness values before and after Diamondyzing.
![]()
Using their close links to the motor sport industry, Warren and Hupe have coated engine pistons and rings, and tested these, in a standard US 5 litre auto engine, coupled to a dynamometer. Thanks to reduced frictional loss, an approximately 6 % increase in both horsepower and torque was measured, and the implications for potential fuel savings are obvious. After testing, the engine was dismantled and pistons and rings were found to be in pristine condition. Figure 3shows a piston after 6 hours on full load. Note very slight wear (as loss of colour) at the bottom right corner.

But, as the inventors see it, the greatest strength of the new process lies in the superb corrosion resistance it imparts. Results of salt spray tests, as per ASTM B117, are shown in the table below. In addition to use of standardised B117 testing, anodised samples have also been exposed to an in-house variation of B117, whereby the cabinet is operated for 12 hours per day, with samples resting overnight in the cabinet, exposed to air. Comparison of the two tests showed, as might be expected, that the latter is more severe, on the basis that counted hours are only for spray in operation, and exclude rest periods. Properties of the new coating are summarised in Table 1, where it should be noted that endurance values (hours) in the salt spray tests are not, as is normally the case, hours to failure, but rather (in the first case) because after absence of any traces of corrosion, it was felt pointless to continue past 8 400 hours. In the second case, the tests continue, and the figure 2 000 hours represents the present state of play again with no evident corrosion so far.

| Table 1: Some Properties of Diamondyze Anodised Aluminium | |
|
Drop Impact Test ASTM D2794 |
2lb weight dropped 16” causes no damage. Untreated sample showed 0.149” indentation, treated sample 0.198”. Untreated sample showed slight stretch marks |
| Gloss | No change of gloss after anodizing |
| Salt spray ASTM B117 | 8 400 hrs on 6061 with no change at end of test. 2 000 hrs on 2024 (unsealed) with no change & both tests therefore halted |
| Chemical resistance | 24 hour immersion in alcohol/MEK mixture & aircraft paint stripper (contains methylene chloride) shows no effect. 2 month soak in brake fluid showed no attack. |
|
OvenOff* (*Strongly alkaline proprietary Oven Cleaner)
|
12 hours, no effect |
| Thermal resistance | Approx 10 % reduction in heat transfer (thermal resistance, as insulator) |
| Surface roughness (Ra) | Prior to Type II Diamondyze anodising, 6061 test panel had roughness 2.791. After anodising, Ra = 1.782 |
| Ford APGE Accelerated corrosion test (FLTM B1 123-01) | 285 cycles as of 1st Oct 2012 (Pass is 40 cycles) |
| Gravelometer (GM) | 5A (see Figure 4) |
| Resistance to grit blast | Using 120 mesh alumina grit at 120 psi, minimal removal of oxide observed. Behaviour similar to hard chromium plating, with grit particles starting to glow red hot |
| Accelerated salt spray | Over 5 400 hrs on 6061 alloy as of Sept 1st 2012 and ongoing |
| Humidity test | 18,000 hrs on 6061 with no change as of Sept 2012 |
Another dramatic demonstration of Diamondyze coating hardness is the application of a metal file to the coating. The file teeth are damaged rather than the coating. A further feature concerns the dimensional changes after anodising, a key issue where precision engineering components are involved. The aluminium oxide formed by anodising grows partly outwards from the original surface, partly inwards. In conventional anodising, the ratio of outward to inwards is typically 70 % inwards, 30 % outwards. In the case of Diamondyzing , a much smaller outward growth is reported, often as little as 5 % with up to 95 % of growth being inwards.
Corrosion and Wear Resistance
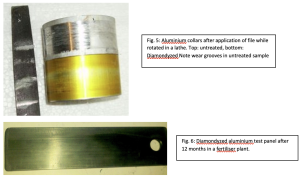
Numerous tests confirm the exceptional properties of the new coating. In what follows, it should be noted that while use of dyestuffs to colour anodised samples has no significant effect on their wear or corrosion resistant properties, it can act as a useful “tell-tale” since any loss of coating by corrosion or wear, also removes the outer colouring. In fact, presence of dyestuffs slightly diminishes wear and corrosion performance of these coatings. In a Taber Abraser, 40 000 cycles were completed with no visible wear or weight loss. In another test, two aluminium collars, one as-received, the other Diamondyzed anodised, were mounted in a lathe and, while being rotated, a hand-held file was applied. The results are seen in Figure 5, with the grooved surface of the untreated sample contrasted with the almost pristine dyed and treated sample. In terms of corrosion, Figure 6 shows a Diamondyzed aluminium test panel exposed for one year in an inorganic fertiliser plant constructed entirely of stainless steel. The stainless steel screw used to attach the test piece was itself corroded but the test panel itself remain intact.
Further Developments
Such benefits as might be obtained from anodising automotive engine pistons with the new approach would be further increased by similarly anodising the cylinder bores of aluminium engine blocks, and trials of this are in progress. Initial success has also been achieved in bonding dry film lubricants and thermal barrier coatings onto Diamondyze surfaces (as would be required to optimise engine performance). Resin-based clearcoats have also been applied without problems, all of these offering improved increased engine performance in terms of thermodynamics and lubricity.
On the basis of information provided, Diamondyze certainly appears to be a significant advance in anodising technology. Its protagonists envisage it, in many applications, as a lower-cost and lighter-weight alternative to stainless steel. In broad terms, the claims made for Diamondyze have been endorsed by Metal Finishing companies and in-house Finishers now using the process. How novel is the process? Without a better knowledge of the chemistry, it is hard to say, but it is the case that there is a long history of impregnating the porous alumina structure of anodised aluminium with electrodeposited metal (as in electrocolouring), with dyestuffs and with finely divided polymers, notably PTFE, as described in [4]. Further developments of this process will be keenly followed.
References
[1] Quicktime Video at www.camcoat.com/diamondyze
[2] Tech Line Coatings. www.techlinecoatings.com
[3] Hupe Manufacturing www.hupemfg.com
[4] A.T.Kuhn Impregnation of anodised aluminium. Metal Finishing (19XX) Bd XX ss. xxxx
